Research
COMPREHENSIVE PROTEOMICS
Proteins are a cell’s machinery and perform most functions within living organisms. Many illnesses and diseases are caused by problems that occur at the protein level and understanding what goes wrong at the protein level will help future disease prevention, diagnosis, and treatment. Proteomics seeks to understand the role of proteins in living systems and begins with the systematic identification and quantitation of as many proteins as possible from a cell, tissue, organism, or biological community (e.g. a microbiome) or other sample. A goal of comprehensive proteomics is to measure all the proteins present in a sample, but this is currently not possible due to the complexity of the samples and limitations of measurement technologies. The factors that contribute to the complexity of proteomes are numerous while key factors that contribute to complexity include: the large number of proteins present in an organism at any point in time, protein concentrations changes with time, most proteins have numerous modification states, and the dynamic range of protein relative abundance is huge. We focus on the development of new microfluidic and nanofluidic systems to improve comprehensive proteome analysis for mass spectrometry (MS) based proteomics. A goal of our systems is to integrate sample preparation and separation into microfluidic/nanofluidic systems before introducing into the MS by electrospray ionization (ESI). Once fully developed, these systems will increase the number of proteins identified providing a more complete description of the proteome while increasing the speed and reproducibility.
NANOFLUIDIC/MICROFLUIDIC INTERFACES (NMIs)
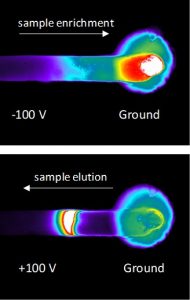
One functional module of these larger microfluidic systems are nanofluidic/microfluidic interfaces (NMIs) that provide rapid enrichment of proteins and peptides on the microfluidic chip. We have investigated the underlying fundamentals of transport within the NMIs to use them within our larger microfluidic system and ultimately improve the detection of proteins and their various proteoforms. An unique aspect of our NMI concentrators is that they enrich samples based on electrophoresis and are compatible with microchannel coatings that reduce surface charge and deleterious protein adsorption. In the images on the left, the enrichment of a sample that is not detectable at its initial concentration is shown in a microchannel terminated with a nanocapillary membrane. The negatively charged green fluorescent protein sample is enriched by electrophoresis, and an enriched band is eluted by switching the direction of the electric field. The images were acquired on an inverted fluorescent microscope, viewing the microfluidic device from the bottom, with white showing the highest protein concentration and blacking showing regions with near zero protein concentration.
LOW FREQUENCY AC ELECTROPHORESIS
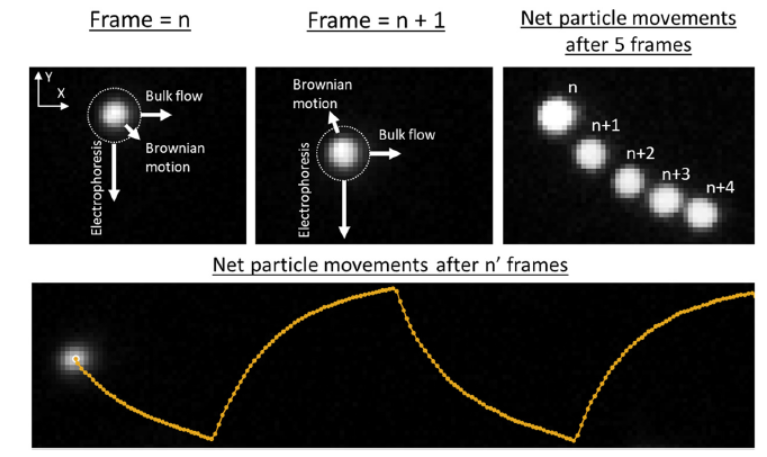
We are developing low-frequency AC electrophoresis microfluidic systems for medical diagnostics and research tools. As diagnostic tools, these next-generation systems will provide earlier detection, low cost, and operate in remote areas. As research tools, they are anticipated to provide improved separations and improved abilities for single-cell phenotyping. Currently, we are developing:
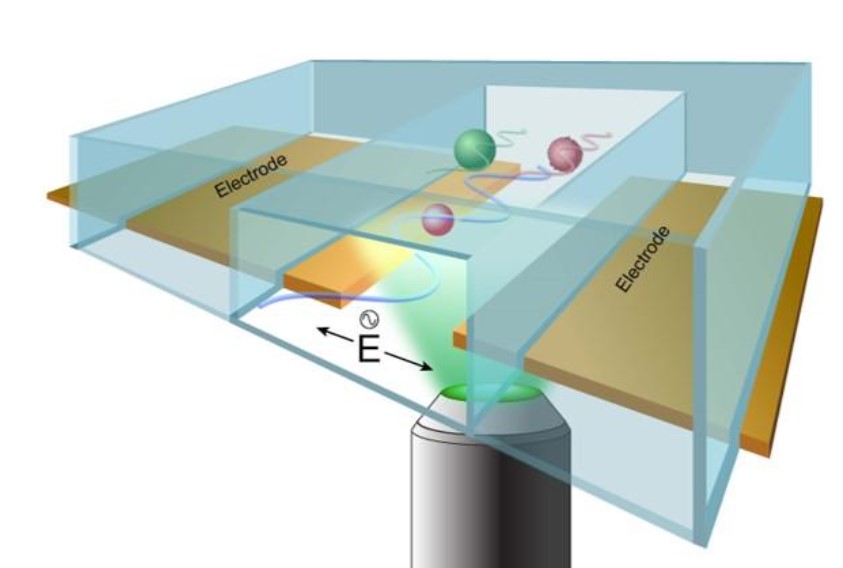
1) Transverse AC electrophoresis for single-cell phenotyping and characterization ofreceptors and ligands on the surface of single cells.
2) Traveling-wave electrophoresis for molecular separations with enhanced control and improved portability.